The Only FDA-Approved Hepatobiliary Specific Contrast Agent (HBA) for MRI Liver Imaging1,2
Eovist® is the only FDA-approved Hepatobiliary Specific Contrast Agent (HBA) for MR liver imaging.1,2 It is a bi-phasic contrast agent combining dynamic phase imaging (similar to extracellular agents) along with functional, hepatobiliary phase information3 to support your comprehensive evaluation of focal liver disease.

Indication
Eovist® is indicated for intravenous use in magnetic resonance imaging (MRI) of the liver to detect and characterize lesions in patients with known or suspected focal liver disease.
How Eovist®, a hepatobiliary agent,1 works
- Following injection, Eovist® is equally eliminated through the renal and hepatic systems
- Eovist® is initially distributed in the extracellular space, allowing for arterial, portal venous, and transitional imaging phase2,3
- Further assessment of lesions through hepatobiliary phase imaging at 20 minutes post injection:
- Lesions with little or no hepatobiliary function will generally not accumulate Eovist®
- Lesions with normal hepatobiliary function will accumulate Eovist®

References: 1. Melanie K. Seale. Hepatobiliary-specific MR Contrast Agents: Role in Imaging the Liver and Biliary Tree. Radiographics.rsna.org; 2009. 2. Eovist [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals; 2018. 3. American College of Radiology CT/MRI Li-RADS 2018 CORE. 4. Zech CJ, Hermann KA, Reiser MF, Shoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent GD-EOB-DTPA. Magn Reson Med Sci. 2007;6:43–52.
Accordion header
In clinical trials among 1,989 patients, of whom 1,581 received the recommended dose:
- Overall, 4% of subjects reported one or more reactions during a follow-up period that for most subjects extended 24 hours after Eovist® administration. The adverse reactions were predominantly of mild to moderate severity.
- The most frequent (≥0.5%) adverse reactions associated with the use of Eovist® were nausea, headache, feeling hot, dizziness, and back pain.

Accordion header
Dynamic and functional liver imaging in <30 minutes1
Eovist® MRIs don't have to require extended table time.
Eovist® imaging protocols allow for the collection of dynamic and functional information in under 30 minutes,1 that is important to your department and patient scheduling needs.


References: 1. Eovist® [package insert]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2018. 2. American College of Radiology CT/MRI Li-RADS 2018 CORE. 3. Jhaveri K, Cleary S, Pascale A. Consensus Statements From a Multidisciplinary Expert Panel on the Utilization and Application of a Liver-Specific MRI Contrast Agent (Gadoxetic Acid). Am J Roentgen AJR. 2015;204:498-509. 4. Yoshioka H, Takahashi N, Yamaguchi M. Double Arterial Phase Dynamic MRI with Sensitivity Encoding (SENSE) for Hypervascular Hepatocellular Carcinomas. J Magn Reson Im. 2002;16:259-266. REF-US-28431. 5. Pietryga JA, Burk LMB, Marin D Respiratory Motion Artifact Affecting Hepatic Arterial Phase imaging with Gadoxetate Disodium. Radiology. 2014;271(2):426-34. REF-US-28430. 6. Manojkumar Saranathan, Dan W. Rettmann. Differential Subsampling with Cartesian (DISCO). J Magn Reson Im 35:1484-1492 (2012).
Accordion header
Uses less Gadolinium Per Dose Than Extracellular MRI Agents

The recommended dose of Eovist® is 0.1mL/kg body weight (0.025 mmol/kg body weight).
Accordion header
See Eovist in action with the case studies below or additional cases linked here. Or go to the Resources section to see all videos.
For The Detection and Characterization of Focal Liver Disease
Detecting Focal Liver Lesions:
MRI with Eovist®
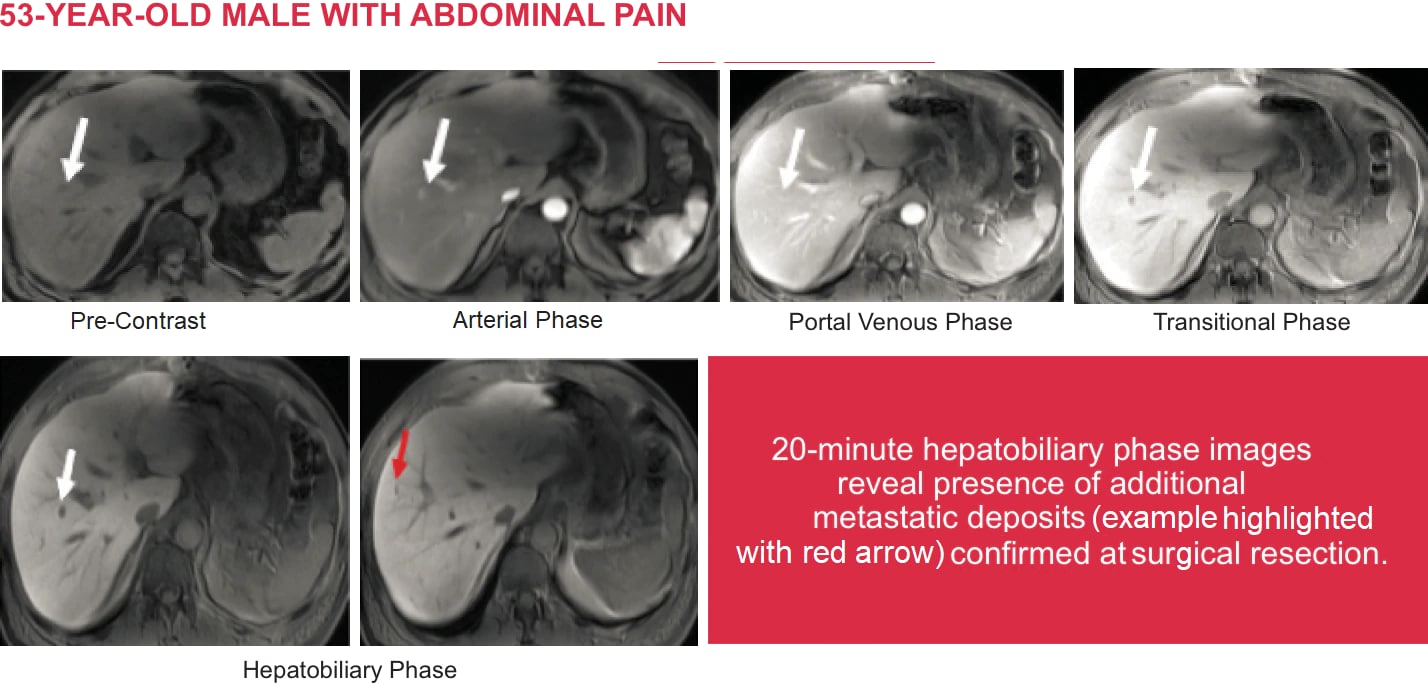
Case courtesy of Rajan T. Gupta, M.D., Associate Professor of Radiology, Duke University Medical Center, Duke University, Durham, North Carolina
The patient data that appears in this document is actual health information but all personal identifiers have been removed or otherwise anonymized. No personally identifiable information is shown.
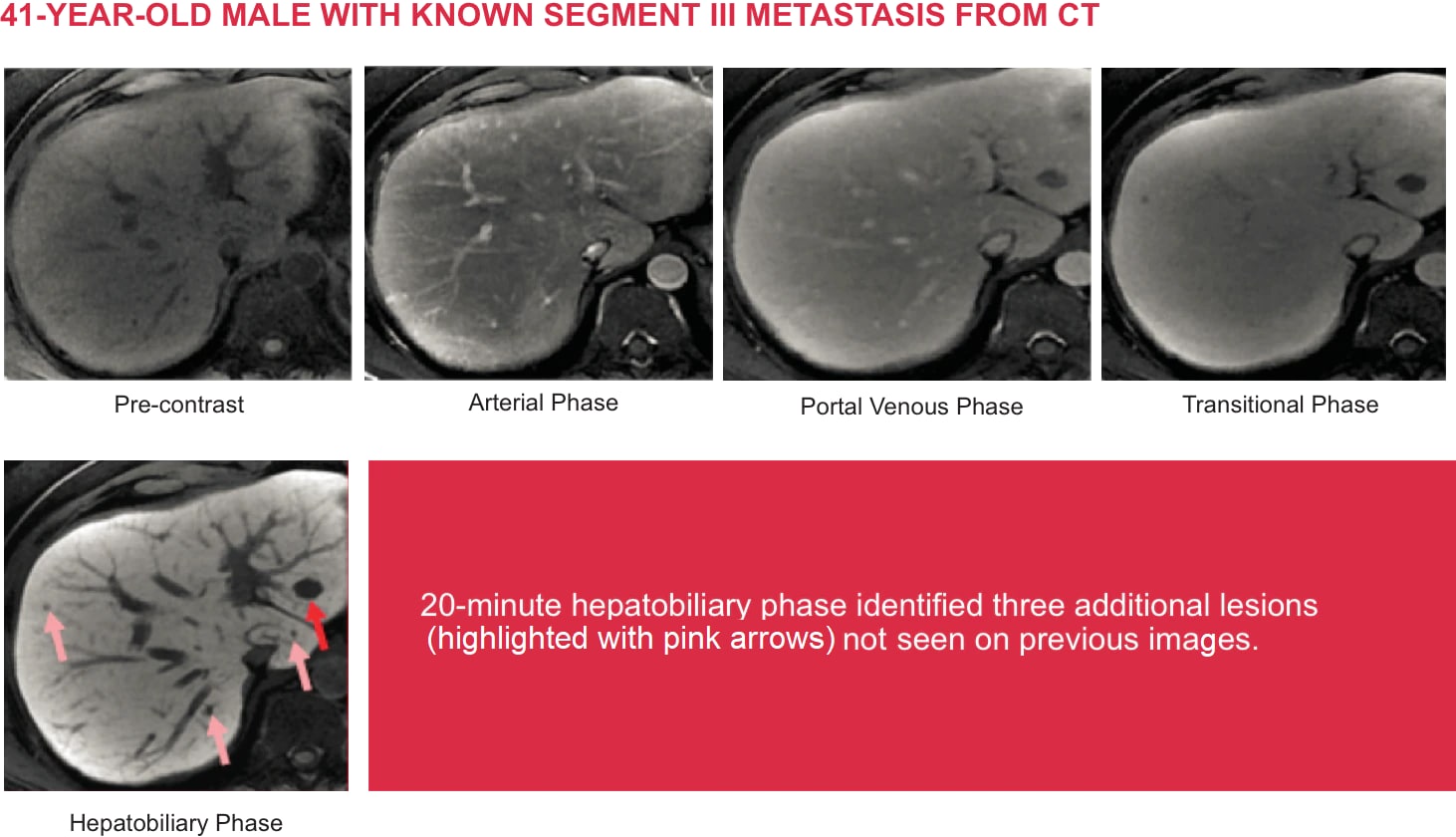
Case courtesy of Dr. Claude Sirlin, University of California San Diego Liver Imaging Group.
Characterizing Lesions with Eovist®
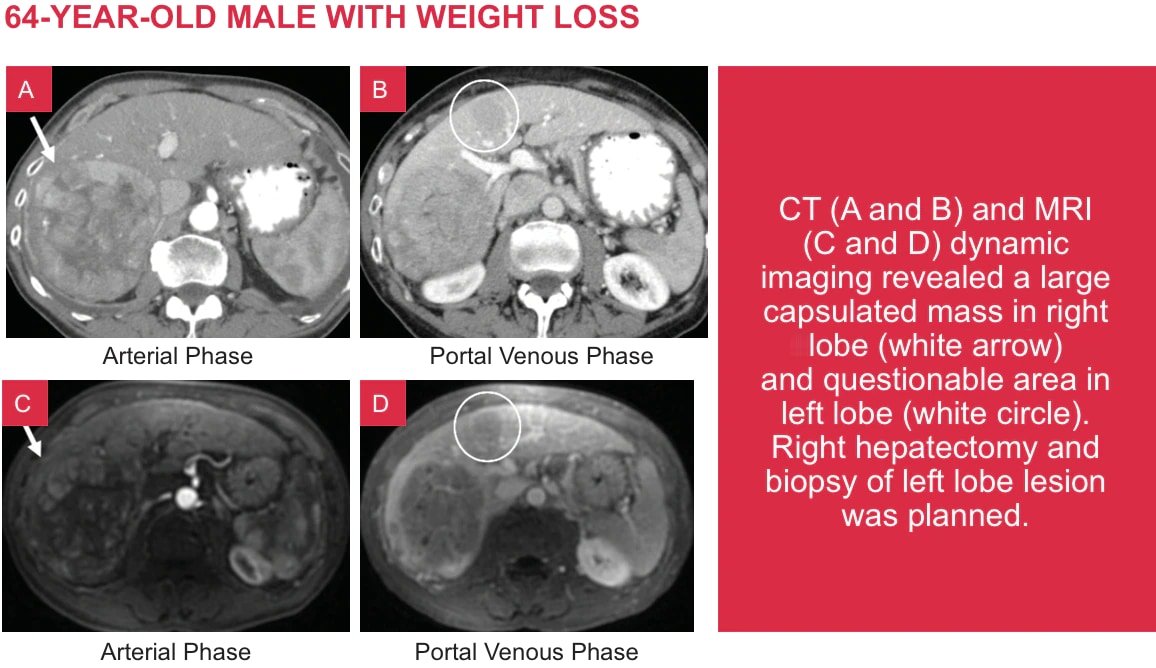
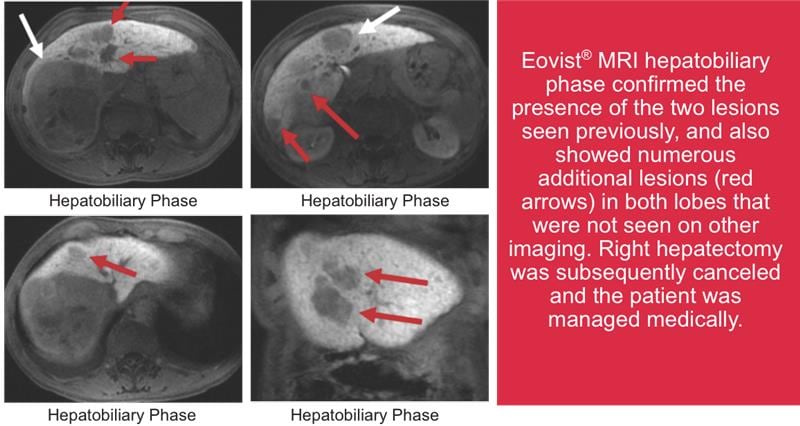
Case courtesy of Dushyant V. Sahani M.D., Director of Computed Tomography, Massachusetts General Hospital and Associate Professor of Radiology. Harvard. Boston, Massachusetts.
Accordion header
Eovist® Packaging
- Eovist® injection is supplied in single-dose, rubber-stoppered vials containing 181.43 mg/mL of gadoxetate disodium, equivalent to 0.25 mmol/mL
- The recommended dose of Eovist® injection is 0.025 mmol/kg body weight

For more information on reimbursement and purchasing options, click here.

