Indications
As a proven X-ray contrast medium with 40 years of experience behind it, Ultravist® has a broad range of indications for intra-arterial and intravenous uses:
- Intra-arterial Procedures*
- Cerebral arteriography and peripheral arteriography in adults
- Coronary arteriography and left ventriculography, visceral angiography, and aortography in adults
- Radiographic evaluation of cardiac chambers and related arteries in pediatric patients aged 2 years and older
- Intravenous Procedures*
- Excretory urography in adults and pediatric patients aged 2 years and older
- Contrast Computed Tomography (CT) of the head and body (intrathoracic, intra-abdominal, and retroperitoneal regions) for the evaluation of neoplastic and non-neoplastic lesions in adults and pediatric patients aged 2 years and older
- Contrast mammography to visualize known or suspected lesions of the breast in adults, as an adjunct following mammography and/or ultrasound
*Specific concentrations and presentations are recommended for each type of imaging procedure [See Dosage and Administration in Full Prescribing Information (sections 2.2, 2.3, 2.4)].
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE
Intrathecal administration, even if inadvertent, may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Ultravist® is not approved for intrathecal use.
.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect or predict the rates observed in practice.
The common adverse reactions reported in >1% of patients in clinical studies with ULTRAVIST are shown in Table below.

.
- The recommended doses for intra-arterial procedures in adults are shown in Table 1.
- Inject at rates approximately equal to the flow rate in the vessel being injected.
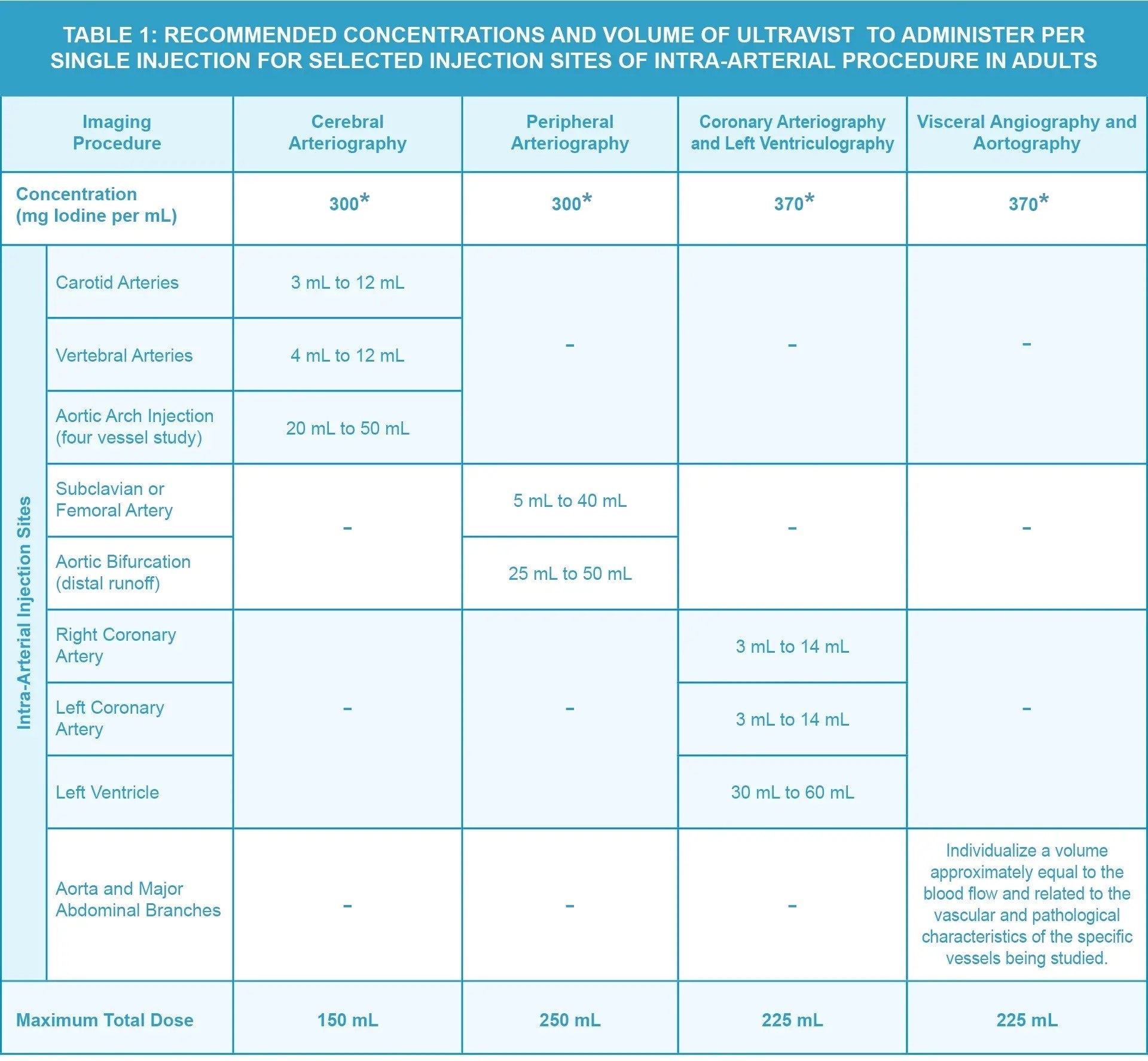
*Use single-dose vials.
.
- The recommended doses for Intravenous procedures in adults are shown in Table 2.
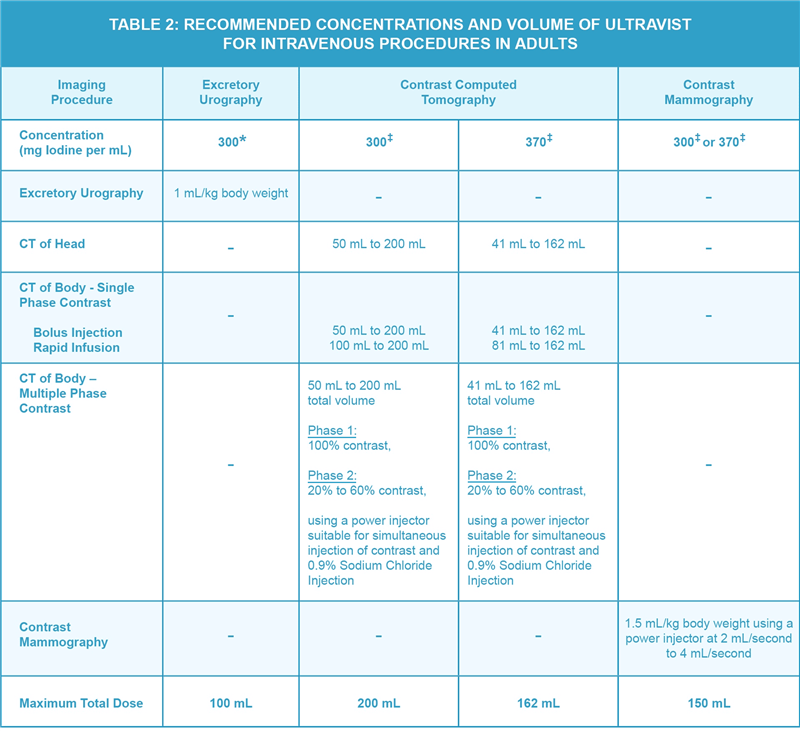
*Use single-dose vials.
‡Use single-dose vials or imaging bulk package.
.
The recommended doses in pediatric patients aged 2 years and older are shown in Table 3.
Safety and effectiveness of ULTRAVIST have not been established in pediatric patients younger than 2 years for radiographic evaluation of cardiac chambers and related arteries, excretory urography, and contrast computed tomography of head and body.
Safety and effectiveness of ULTRAVIST for cerebral arteriography, peripheral arteriography, coronary arteriography and left ventriculography, visceral angiography, aortography, and contrast mammography have not been established in pediatric patients.
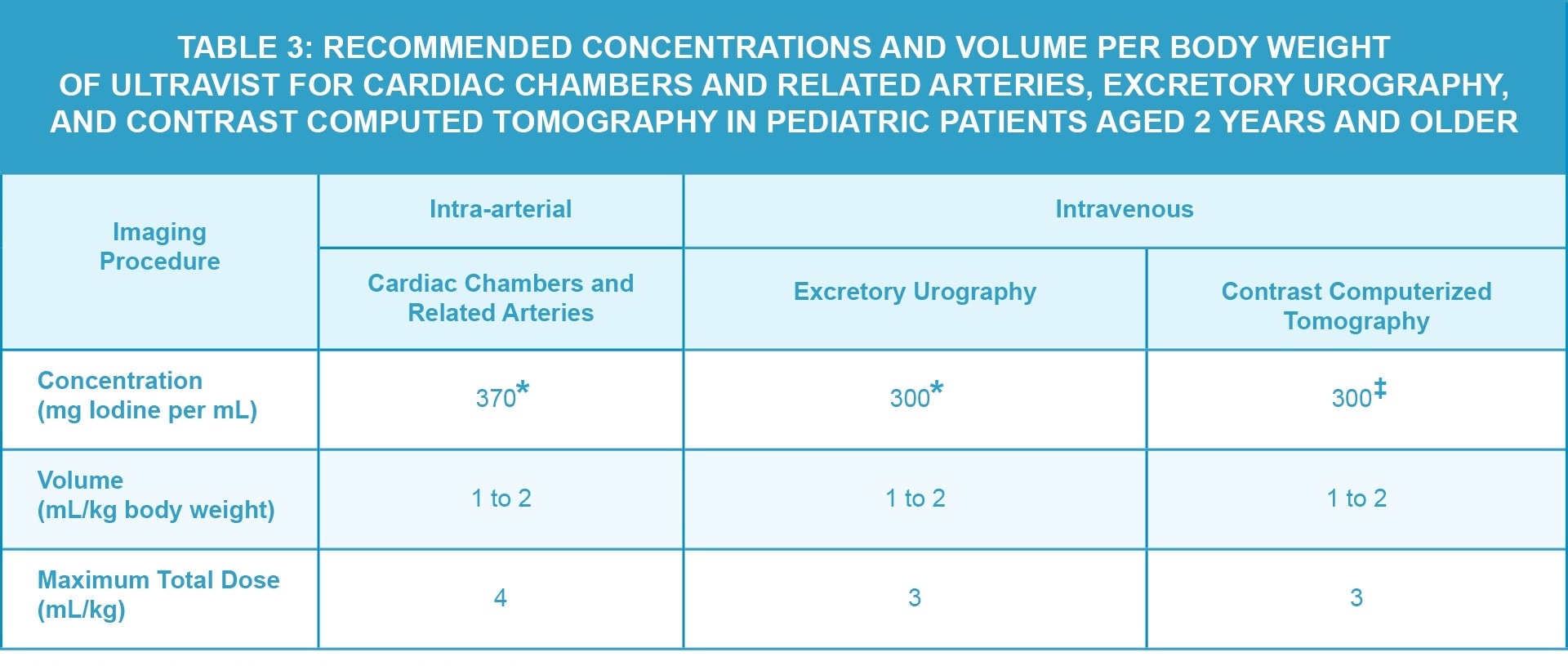
*Use single-dose vials.
‡Use single-dose vials or imaging bulk package.
.
ULTRAVIST is a sterile (sterilized by autoclaving), clear, colorless to slightly yellow, odorless, pyrogen-free aqueous solution available in two concentrations:
- 300 mg Iodine per mL provides 623.4 mg/mL iopromide
- 370 mg Iodine per mL provides 768.86 mg/mL iopromide
Important Dosage and Administration Information
- ULTRAVIST is for intra-arterial or intravenous use only and must not be administered intrathecally [see Warnings and Precautions (5.1)].
- Specific concentrations and presentations of ULTRAVIST are recommended for each type of imaging procedure [see Dosage and Administration in Full Prescribing Information (sections 2.2, 2.3, 2.4)].
- Hydrate patients, as appropriate, prior to and following the administration of ULTRAVIST [see Warnings and Precautions (5.3)].
- Individualize the volume, concentration, and injection rate of ULTRAVIST according to the specific dosing tables [see Dosage and Administration in Full Prescribing Information (sections 2.2, 2.3, 2.4)]. Consider factors such as age, body weight, size of the vessel, and the rate of blood flow within the vessel; also consider extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- Visually inspect ULTRAVIST for particulate matter and/or discoloration, whenever solution and container permit. Do not administer ULTRAVIST if particulate matter (including crystals) and/or discoloration is observed or if containers are defective.
- Use aseptic technique for all handling and administration of ULTRAVIST.
- Warm ULTRAVIST to body temperature before administration.
- ULTRAVIST can be used with 0.9% Sodium Chloride Injection in a power injector suitable for simultaneous injection of contrast [see Dosage and Administration in Full Prescribing Information (section 2.3)]. However, do not mix or inject ULTRAVIST in intravenous administration lines containing other drugs or total nutritional admixtures.
- Discard any unused portion remaining in the single-dose container following initial use.

.
Ultravist® Injection is available as a stable, ready-to-use aqueous solution of iopromide in vials or bottles in concentrations of 300 mg Iodine per mL, and 370 mg Iodine per mL. Ultravist® is available in a number of vial sizes, including the 500 mL Imaging Bulk Package.
- Color-coded vials for easy identification of concentration
- Integrated hanger labels
- Clear glass vials/bottles for examination of product clarity
- Peel-off product identification labels
- Imaging Bulk Package offers 10-hour stand time, allowing flexibility to use doses as needed
- Imaging Bulk Package is only to be used in a room designated for radiological procedures that involve intravascular administration of a contrast agent
For more information on reimbursement and purchasing options, click here.

Related Products
Note: The Bayer in Radiology contrast and device products should be used in accordance with the Prescribing Information and Instructions For Use, respectively.
Related Products
Note: The Bayer in Radiology contrast and device products should be used in accordance with the Prescribing Information and Instructions For Use, respectively.